Other Monoclonal Antibodes
Casirivimab, Imdevimab and Sotrovimab are US FDA approved recombinant neutralizing human IgG1 monoclonal antibodies that target the receptor binding domain of the spike protein of SARS-CoV-2. Whilst not yet evaluated and NICE approved in UK, they may become available for use in the near future; updates to follow.
COVID Sarilumab
(IL-6 inhibitor)
Definition
Invasive mechanical ventilation: any method of controlled ventilation delivered through a translaryngeal or tracheostomy tube, or other methods as defined by the Intensive Care National Audit & Research Centre definition of ‘advanced respiratory support’.
Conditional recommendation
Consider sarilumab for adults in hospital with COVID-19 only if tocilizumab cannot be used or is unavailable. Use the same eligibility criteria as those for tocilizumab. That is, if all of the following apply:
- they are having or have completed a course of corticosteroids such as dexamethasone, unless they cannot have corticosteroids
- they have not had another interleukin-6 inhibitor during this admission
- there is no evidence of a bacterial or viral infection (other than SARS-CoV-2) that might be worsened by sarilumab.
And they either:
- need supplemental oxygen and have a C-reactive protein level of 75 mg/litre or more, or
- are within 48 hours of starting high-flow nasal oxygen, continuous positive airway pressure, non-invasive ventilation or invasive mechanical ventilation.
In April 2021, the marketing authorisations for sarilumab do not cover use in COVID-19. See
NICE’s information on prescribing medicines for more about off-label and unlicensed use of medicines.
The recommended dosage for sarilumab is a single dose of 400 mg by intravenous infusion.
For sarilumab use in pregnancy, follow the Royal College of Obstetrics and Gynaecology guidance on coronavirus (COVID-19) infection and pregnancy.
For full details of adverse events and contraindications, see the summaries of product characteristics.
See NHS England’s Interim Clinical Commissioning Policy on sarilumab for critically ill patients with COVID-19 pneumonia (adults) for further information.
COVID-19 rapid guideline: managing COVID-19 (NG191)
© NICE 2021. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-andconditions#notice-of-rights). Last updated 3 June 2021
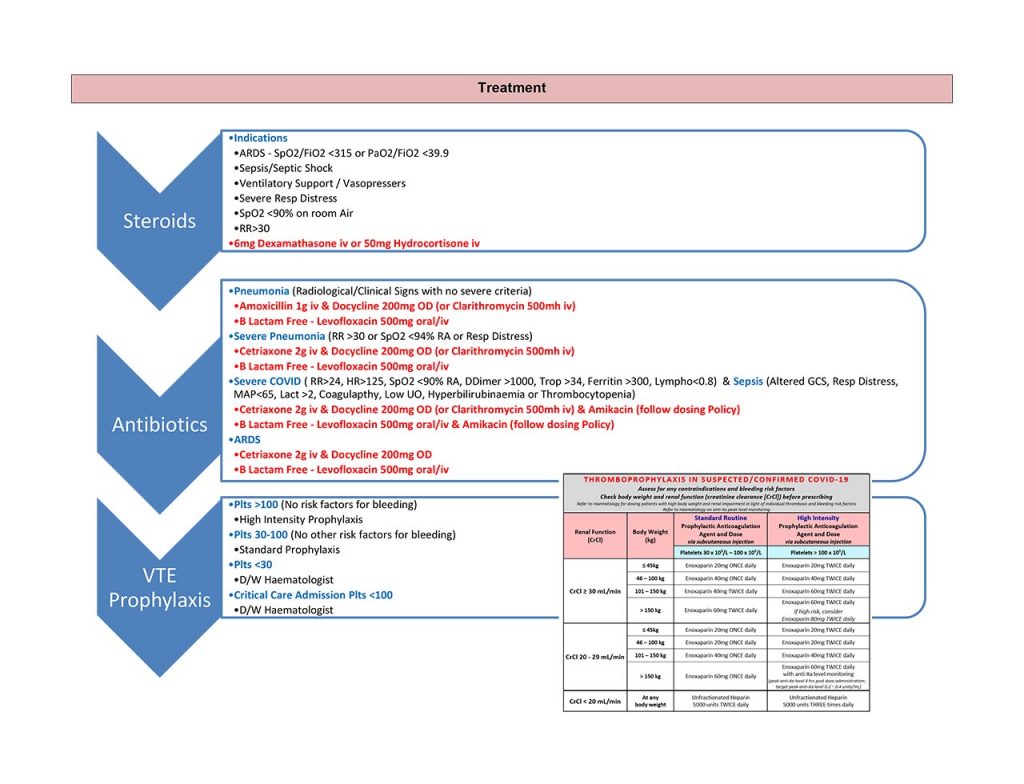
Steroid Immunosuppression
Steroids should be considered standard of care and immediately started in cases that meet hospital admission criteria, usually Severe COVID Pneumonitis.
There is body of evidence to support its use in cases showing early ARDS, septic shock and confers mortality benefit.
What: Dexamethasone 6mg PO/IV once daily (with STAT dose); or
Prednisolone 40mg PO once daily; or
Hydrocortisone 50mg IV three times daily.
Administration: Oral (PO) is the preferred route, intravenous (IV) only when preferred route is not available.
Duration: 10 days.
Cautions
Already using long-term steroids?
Where patients are already on steroids (usually prednisolone) it is reasonable to convert to dexamethasone, but remember to return back to maintenance dose after 10 days.
Hyperglycemia
Steroid induced raised blood sugars are a consequence of high dose steroid use. It does not imply diabetes, although diabetic patients are at greater risk of elevated blood sugar measurement.
It is crucial to monitor and manage excessive blood sugar concentrations.
Each trust has developed guidelines for Hyperglycemia in COVID Steroid administration, please refer to you own trust guidelines for more information.
Venous Thromo-embolic Prevention (VTE)
COVID Tocilizumab
(IL-6 inhibitor)
COVID Remdesivir
(Viral RNA polymerase inhibitor)
Anti-COVID therapy is a fast and dynamic changing landscape, consideration should be given to clinical suitability and eligibility to newer classes of drugs.
Last updated: December 2020.
Anti-COVID Therapies
COVID Microbial Therapy Review (24-48 hours)
Review of progress and improving clinical state should include further rationalisation of unnecessary medications including antibiotics if ‘Procalcitonin’ is negative and clinical state deems appropriate..
Last updated: December 2020.
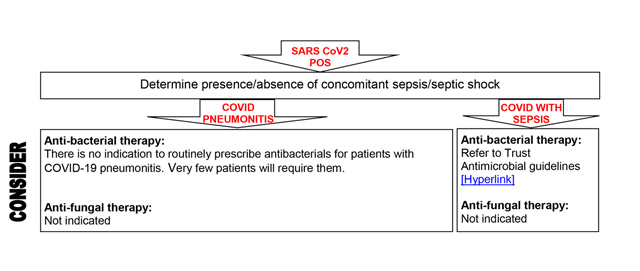
Antibiotics
Consideration should be given to rationalising antibiotic prescriptions in accordance with good antimicrobial stewardship.
Data from the Chelsea and Westminster cohort and nationally/internationally suggest less than 10%, and probably more than 5% have bacterial/fungal pulmonary co-infection on presentation. Some patients can (rarely) present with COVID-19 plus another systemic infection/sepsis.
Prescribe according to local current guidelines.
Last updated: December 2020.
COVID In-Hospital Treatment